Unit Processes
Definition: Unit processes are chemical changes or reactions involved in the conversion of raw materials into desired products. These steps involve chemical transformations.
Focus: The focus is on the chemical reaction and the conditions required for it, such as temperature, pressure, catalysts, and reaction mechanisms.
Examples in Inorganic Industries:
•Ammonia synthesis (Haber-Bosch process): N2+3H2→2NH3
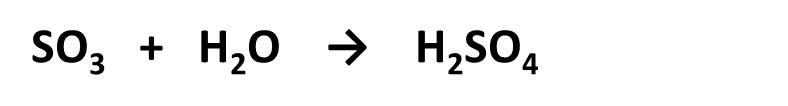
•Sulfuric acid production (Contact process): 2SO2+O2→2SO3
•Chlor-alkali process: Production of Cl2, H2 and NaOH via electrolysis of brine.
Unit Operations
Definition: Unit operations involve physical processes used in handling, separating, mixing, and purifying materials. These do not change the chemical composition of substances but deal with their physical form or state.
Focus: The focus is on the physical manipulation of materials, ensuring efficient flow and separation during production.
Examples in Inorganic Industries:
•Filtration: Removing impurities from brine in the chlor-alkali process.
•Distillation: Purifying liquid products or solvents.
•Crystallization: Producing solid salts like sodium chloride or potassium nitrate.
•Grinding and milling: Reducing the particle size of ores or catalysts.
•Heat exchange: Cooling or heating reactants/products in reactors.
Integration in Industries
In the inorganic process industries, unit processes and unit operations are integrated into the production workflows. For instance:
•Ammonia Production:
•Unit Process: Synthesis of ammonia from nitrogen and hydrogen.
•Unit Operation: Separation of ammonia from unreacted gases through condensation.
By combining these concepts, industries design efficient and sustainable processes for manufacturing inorganic chemicals and materials.
Process Designs
In the inorganic process industries, the process design is focused on the development, optimization, and implementation of processes for manufacturing of inorganic chemicals and materials.
Steps in Process Design for Inorganic Industries:
1.Raw Material Selection: Identify and evaluate sources of raw materials (e.g., ores, brines, gases).
●
2.Reaction Pathway Design: Determine chemical reactions, catalysts, and reaction conditions.
●
3.Flowchart and Process Flow Diagram: Map out material and energy flows through the system.
4. Equipment Selection: Design reactors, separators, heat exchangers, and storage systems.
5. Mass and Energy Balances: Calculate input or output requirements and evaluate efficiency.
6. Waste Management Systems: Plan treatment or recycling of by-products and effluents.
7. Process Control and Automation: Implement sensors, controls, and feedback systems for precision.
8. Pilot Plant Testing: Build and test small-scale models before scaling up.
9. Economic Evaluation: Estimate costs, profits, and feasibility.
10. Safety Analysis: Assess risks, explosion potential, and corrosion resistance.
Raw Materials
Raw materials are the basic substances obtained from natural sources or industrial by-products that are processed to produce inorganic chemicals and materials.
Categories of Raw Materials in Inorganic Industries:
1.Minerals and Ores:
1.Iron Ore (Fe₂O₃, Fe₃O₄): Used in steel and iron production.
2.Limestone (CaCO₃): Used in cement and lime (CaO) production.
2.Industrial Gases and Elements:
1.Nitrogen (N₂): Ammonia synthesis (Haber process).
2.Hydrogen (H₂): Used in hydrogenation and ammonia synthesis.
3.Salts and Alkalis:
1.Sodium Chloride (NaCl): Electrolysis for chlorine, caustic soda, and hydrogen.
2.Potassium Chloride (KCl): Source of potassium fertilizers.
4. Sulfur and Sulfur Compounds:
1.Elemental Sulfur (S): Sulfuric acid production.
2.Pyrite (FeS₂): Alternative source of sulfur dioxide (SO₂).
5. Water:
1.Used as a solvent, coolant, and reactant in various processes.
6. Industrial Waste and By-Products:
1.Slag and Fly Ash: Utilized in cement and construction materials.
2.Scrap Metals: Recycled in metal refining processes.
Energy Sources
Cement Manufacturing: Coal or petroleum coke for kiln (thermally insulated oven) heating.
Glass Industry: Natural gas for melting and forming (a mixture of hydrogen and nitrogen) processes.
Metallurgy: Electricity for electrolysis, coal or coke for smelting.
Fertilizer Production: Natural gas as a feedstock in ammonia synthesis.
Chemical Synthesis: Hydrogen and synthesis gas (mixture of hydrogen and carbon monoxide) derived from natural gas or coal gasification.
Heat Transfer
Heat transfer involves the movement of thermal energy due to a temperature gradient. It controls reaction rates, phase changes, and energy efficiency in industrial processes.
Conduction: Transfer of heat through solid materials or stationary fluids.
Convection: Transfer of heat by fluid motion (liquids or gases).
Radiation: Transfer of heat via electromagnetic waves without a medium.
Mass Transfer
Mass transfer is the movement of molecules, atoms, or ions from one phase to another, driven by a concentration gradient. It is vital for separations and mixing operations.
Diffusion: Movement of particles from high to low concentration.
Convection: Mass transfer due to bulk fluid motion.
Phase Equilibrium Transfer: Movement between phases, such as liquid-gas or solid-liquid transitions.
Common Terms
Production is the process of creating goods and services using raw materials, labor, and capital to meet market demand.
Consumption refers to the use of goods and services by individuals, households, or industries. It is the end purpose of economic activity.
Market evaluation is the process of analyzing and assessing market conditions to determine demand, supply, competition, and pricing strategies for products or services.
