Essential Plant Nutrients
Macronutrients (Needed in large amounts)
Primary Macronutrients:
Nitrogen (N): Leaf growth, chlorophyll, and protein synthesis.
Phosphorus (P): Energy transfer, root development, and flowering.
Potassium (K): Plant health, disease resistance, and water regulation.
Secondary Macronutrients:
Calcium (Ca): Cell wall structure and root growth.
Magnesium (Mg): Central to chlorophyll and enzyme activation.
Sulfur (S): Protein synthesis and enzyme function.
Micronutrients (required in smaller amounts)
Iron (Fe): Key for chlorophyll synthesis and enzyme function.
Manganese (Mn): Plays a role in photosynthesis and nitrogen metabolism.
Zinc (Zn): Essential for hormone production and enzyme systems.
Copper (Cu): Important for reproductive growth and enzyme activity.
Boron (B): Vital for cell wall formation and pollen tube growth.
Molybdenum (Mo): Helps in nitrogen fixation and conversion of nitrates to ammonia.
Chlorine (Cl): Involved in photosynthesis and osmotic balance.
Nickel (Ni): Important for urease enzyme function.
Natural inorganic fertilizers
Natural inorganic fertilizers are mineral-based fertilizers that provide essential nutrients to plants.
Characteristics:
Derived from non-living, naturally occurring materials.
Mined or extracted from earth.
Contain key nutrients like nitrogen (N), phosphorus (P), and potassium (K).
Difference from Organic Fertilizers: No organic matter; mineral-based.
Rock Phosphate/ phosphorite/ phosphate rock
Source of phosphorus (P).
Mined from ancient deposits.
The general formula for pure rock phosphate is Ca10(PO4)6(X)2, where X is F−, OH− or Cl−
Greensand
Contains glauconite (potassium and iron).
Improves soil structure.
Limestone (Calcium Carbonate)
Raises soil pH, especially in acidic soils.
Provides calcium.

Potash
Source of potassium (K).
Derived from potassium-rich minerals like sylvite.
Gypsum (Calcium Sulfate)
Provides calcium and sulfur.
Improves soil structure, especially in clay soils.
Epsom Salt (Magnesium Sulfate)
Source of magnesium and sulfur.
Important for photosynthesis and metabolism.
Artificial Fertilizers
Artificial fertilizers are synthetic products that are chemically manufactured to supply essential nutrients to plants, typically nitrogen (N), phosphorus (P), and potassium (K).
Characteristics:
Immediate availability of nutrients for rapid plant growth.
Can be tailored to meet specific plant nutrient needs.
Readily available and easy to apply.
Highly concentrated nutrients require smaller application amounts.
Types: Granules, liquids, slow-release formulas.
Nitrogen Fertilizers
•Common form: Urea, Ammonium nitrate.
•Promote leafy growth and photosynthesis.
Phosphorus Fertilizers
•Common form: Superphosphate, Monoammonium phosphate.
•Enhance root development and flowering.
Potassium Fertilizers
•Common form: Potassium chloride, Potassium sulfate.
•Help with drought resistance, fruiting, and disease resistance.
Compound Fertilizers
•Fertilizers that contain two or more of the essential nutrients (NPK).
•Designed for general-purpose use or for specific crops.
Nitrogen Fixation
Nitrogen fixation is the process of converting nitrogen gas (N₂) from the atmosphere into a form that plants can use, such as ammonia (NH₃) or nitrate (NO₃⁻).
Importance:
• Nitrogen is essential for plant growth, especially for protein synthesis and chlorophyll production.
• Plants cannot directly use atmospheric nitrogen, so it must be converted into forms plant can
absorb through their roots.
Forms of Nitrogen in Nature
Atmospheric Nitrogen (N₂):
•78% of the Earth’s atmosphere is nitrogen gas, but it is inert and cannot be used directly by plants.
Ammonia (NH₃) and Ammonium (NH₄⁺):
•Forms that plants can absorb directly from the soil.
Nitrates (NO₃⁻):
•A form of nitrogen produced by bacteria and useful for plant uptake.
Biological Nitrogen Fixation:
•The most important and natural process.
•Carried out by nitrogen-fixing bacteria, often in symbiosis with plants.
Physical Nitrogen Fixation:
•Lightning strikes break nitrogen molecules (N₂) in the atmosphere, forming nitrogen oxides that
mix with rain to create nitrates.
Industrial Nitrogen Fixation:
•The Haber-Bosch process is used to produce ammonia for fertilizers.
Biological Nitrogen Fixation
Bacteria Involved:
•Rhizobium (legumes), Azotobacter, Clostridium, and others.
Symbiosis with Legumes:
•Nitrogen-fixing bacteria live in root nodules of leguminous plants (e.g., peas, beans, clover).
•In exchange for carbohydrates, the bacteria fix nitrogen into ammonia, which plants can use.
Process:
•The bacteria convert atmospheric nitrogen into ammonia using the enzyme nitrogenase.
•The plant provides the bacteria with energy (sugars) produced via photosynthesis.

Nitrogen Cycle
Nitrogen Cycle is a biogeochemical process through which nitrogen is converted into many forms, consecutively passing from the atmosphere to the soil to organism and back into the atmosphere. It involves several processes such as
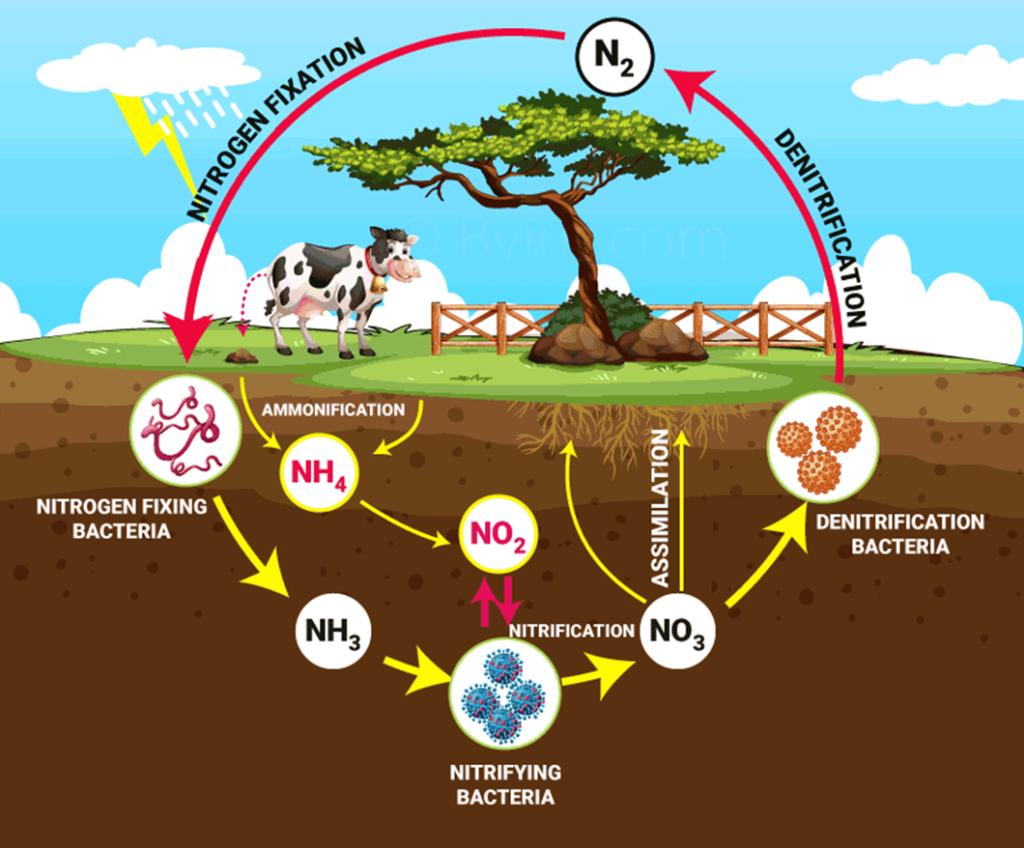
Nitrogen Fixation:
•Conversion of N₂ to NH₃ bacteria.
Nitrification:
•Soil bacteria convert ammonium (NH₄⁺) to nitrate (NO₃⁻), making it available for plant uptake.
Assimilation:
•Plants take up ammonium or nitrate from the soil and use it for growth.
Ammonification (Decomposition):
•Decomposers break down organic matter, releasing nitrogen back into the soil.
Denitrification:
•Some bacteria convert nitrates back into nitrogen gas, returning it to the atmosphere.
Manufacture of Ammonia
The manufacture of ammonia is primarily carried out through the Haber-Bosch process. In this process, the atmospheric nitrogen is converted to ammonia by reacting it with hydrogen. Here a metal catalyst is used and high temperatures and pressures are maintained.
N2(g) + 3H2(g) ⇌ 2NH3(g) ΔH=−92.4 kJ/mol
The reaction is exothermic, therefore lower temperatures theoretically favor ammonia production, but the reaction rate is too slow at lower temperatures.
At higher pressures and moderate temperatures, the reaction reaches a dynamic equilibrium.
Process Conditions:
•Pressure: 150-300 atmospheres
•Temperature: 400-500°C
•Catalyst: Iron-based catalyst, often with promoters like potassium or aluminum oxide to increase efficiency.
The Haber-Bosch process enables the industrial-scale production of ammonia, which is a primary ingredient in nitrogen fertilizers.
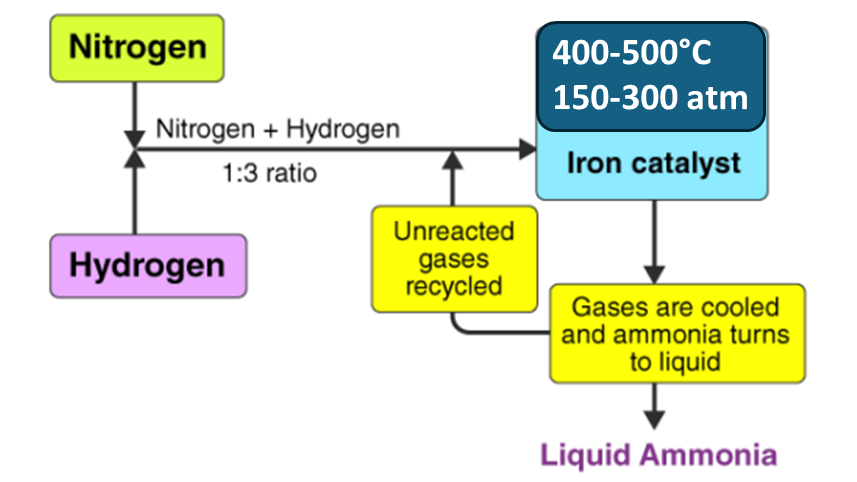
Key Stages:
Nitrogen Extraction: Nitrogen is separated from the air using cryogenic distillation or pressure swing adsorption.
Hydrogen Production: Hydrogen is typically obtained from methane (natural gas) via steam methane reforming.
Ammonia Synthesis: The nitrogen and hydrogen gases are combined in a reactor under high pressure (150-300 atm) and high temperature (400-500°C) in the presence of an iron catalyst.
Ammonia Separation: The ammonia produced is condensed by cooling, separating it from unreacted nitrogen and hydrogen, which are recycled.
Nitrogen Extraction
Air is the primary source of nitrogen, which makes up about 78% of Earth’s atmosphere.
Nitrogen extraction from air is typically done using one of two methods:
•Cryogenic Distillation: Air is cooled to very low temperatures, causing oxygen to liquefy, leaving nitrogen in its gaseous form.
•
•Pressure Swing Adsorption: A system of adsorbent materials is used to selectively capture oxygen and other gases, leaving nitrogen behind. The pressure is then released, and nitrogen is collected.
•
Once separated, nitrogen gas is used in the Haber-Bosch process.
Hydrogen Production
Steam Methane Reforming is the most common method of producing hydrogen in large-scale ammonia production. The process involves reacting methane with steam at high temperatures (700-1000°C), typically with a nickel catalyst:
CH4 + H2O → CO + 3H2
Water-Gas Shift Reaction: The carbon monoxide produced in the first step can further react with water to produce additional hydrogen:
CO + H2O → CO2 + H2
Purification: The hydrogen produced must be purified to remove trace amounts of impurities (such as CO₂), ensuring that only pure hydrogen is fed into the Haber-Bosch reactor.
Hydrogen Production
Steam Methane Reforming is the most common method of producing hydrogen in large-scale ammonia production. The process involves reacting methane with steam at high temperatures (700-1000°C), typically with a nickel catalyst:
CH4 + H2O → CO + 3H2
Water-Gas Shift Reaction: The carbon monoxide produced in the first step can further react with water to produce additional hydrogen:
CO + H2O → CO2 + H2
Purification: The hydrogen produced must be purified to remove trace amounts of impurities (such as CO₂), ensuring that only pure hydrogen is fed into the Haber-Bosch reactor.
Ammonia Separation and Recycling
After ammonia is synthesized, the gas mixture must be cooled to allow ammonia to condense into a liquid state.
Ammonia Condensation: Ammonia has a boiling point of -33°C at atmospheric pressure, so cooling the reaction mixture causes ammonia to liquefy.
Recycling: The remaining gases, primarily unreacted nitrogen and hydrogen, are separated from the ammonia and recycled back into the reaction vessel. This recycling helps maximize the yield of ammonia by ensuring that unreacted gases participate in further synthesis.
Urea
Urea is synthesized through the Bosch-Meiser urea process (also known as the urea process), which involves reacting NH₃ with CO₂ under high pressure and temperature.
The process involves two main reactions:
Formation of ammonium carbamate (NH₄CO₂NH₂):
2NH3(g) + CO2(g) → NH4CO2NH2(aq)
In this reaction, ammonia reacts with carbon dioxide to form ammonium carbamate, which is an intermediate product. This reaction occurs at high pressure (150-250 atm) and moderate temperature (150-250°C).
Dehydration of ammonium carbamate to urea:
NH4CO2NH2(aq) → NH2CONH2(aq) + H2O
The ammonium carbamate is then heated to remove water, producing urea. This step is carried out at temperatures around 180-200°C.
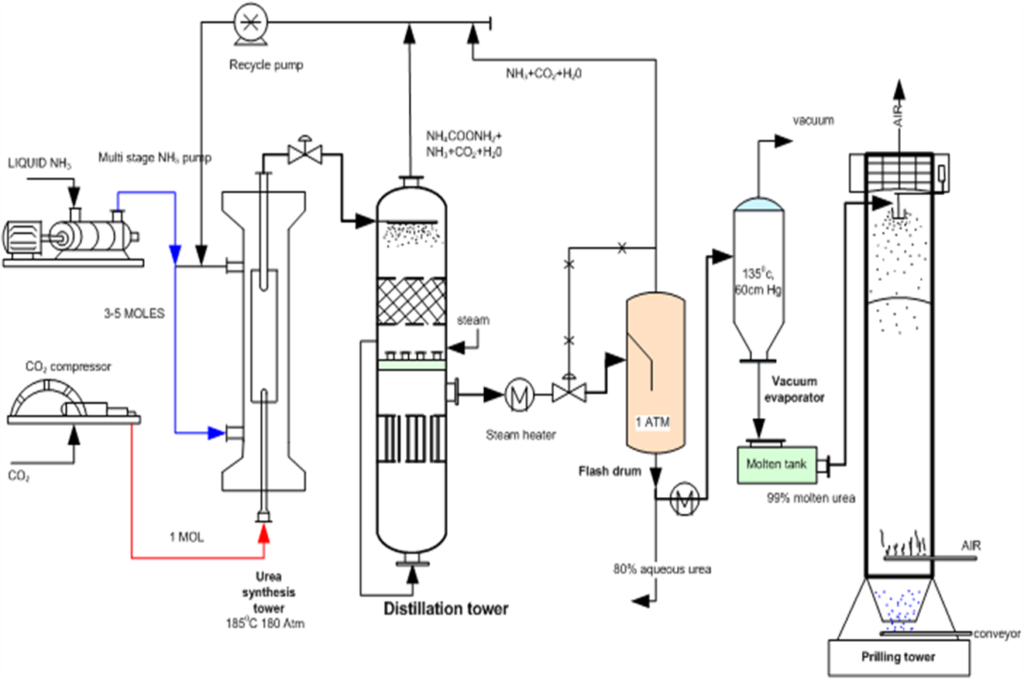
Urea Recovery and Purification:
Urea is separated from the reaction mixture by cooling and distillation processes. Urea is highly soluble in water. It is first separated from any excess ammonia and carbon dioxide in the reactor.
Urea is then purified through evaporation to remove remaining impurities. The final product is concentrated urea solution, which can be further processed into solid urea (through prilling or granulation) or used directly in liquid fertilizer form.
Ammonium sulfate
Ammonium sulfate is widely used as a fertilizer due to its dual benefits of providing nitrogen (N) and sulfur (S), which are essential nutrients for plant growth.
Key Features as a Fertilizer
1.Nitrogen Content:
1.Contains 21% nitrogen in ammonium (NH₄⁺) form, which is readily available to plants.
2.Promotes lush, green vegetative growth, especially in nitrogen-demanding crops like corn, wheat, and rice.
2.Sulfur Content:
1.Supplies 24% sulfur, a critical nutrient for protein synthesis, enzyme function, and chlorophyll production.
2.Sulfur deficiency is common in many soils, making ammonium sulfate an ideal fertilizer in such conditions.
3.Soil pH Adjustment:
1.Slightly acidic, making it beneficial for crops growing in alkaline soils.
2.Helps lower soil pH over time, enhancing the availability of certain nutrients.
4.Stability and Solubility:
1.Highly stable and soluble in water, ensuring effective delivery to plants
About 90 percent of ammonium sulfate is produced by 3 different processes:
(1)as a byproduct of caprolactam [(CH2)5COHN] production
(2)from synthetic manufacture
(3)as a coke oven byproduct.
The chemical reaction for the synthetic manufacture of ammonium sulfate is:
2 NH3 (g) + H2SO4 (aq) → (NH4)2SO4 (s),
where ammonia gas reacts with sulfuric acid to produce solid ammonium sulfate.
Coke oven gas is an abundant by-product of converting coal into coke used in steel production. But the impurities it contains make it unsuitable for use as fuel or feedstock.
Raw coke oven gas also contains various contaminants, which give coke oven gas its unique characteristics.
•Tar vapors
•Light oil vapors (aromatics), consisting mainly of benzene, toluene and xylene (BTX)
•Naphthalene vapor
•Ammonia gas
•Hydrogen sulfide gas
•Hydrogen cyanide gas
Because of the corrosive nature of ammonia, its removal is a priority in coke oven by-product plants. Historically the removal of ammonia from coke oven gas has yielded one of the more profitable by-products, that of ammonium sulfate.
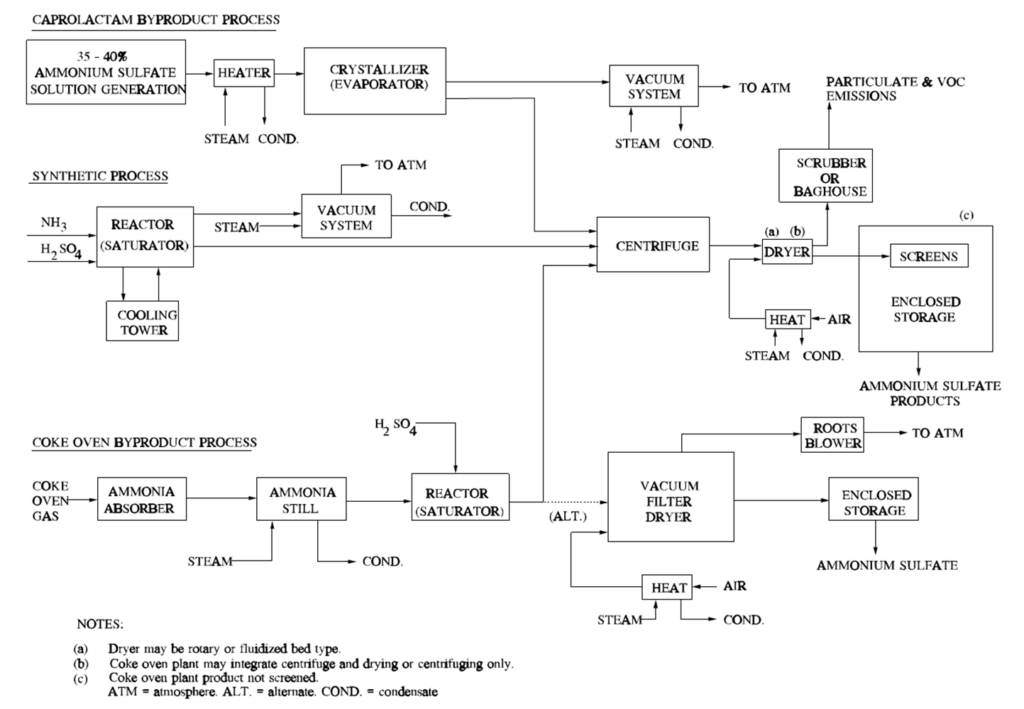
Typical diagram of ammonium sulfate processes.
Ammonium nitrate
Ammonium nitrate is produced by neutralizing nitric acid with ammonia.
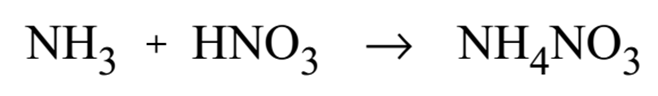
Ammonium nitrate is marketed in several forms, depending upon its use. Liquid ammonium nitrate may be sold as a fertilizer. Solid ammonium nitrate may be produced in the form of prills, grains, granules, or crystals.
Applications
1.Fertilizer:
1.Provides both nitrate (NO₃⁻) and ammonium (NH₄⁺) forms of nitrogen for crops.
2.Commonly used in blended fertilizers or as a standalone product.
3.Some countries ban fertilizer-grade NH4NO3 because it can be used to make bombs.
2.Explosives:
1.Ammonium nitrate is a key ingredient in industrial explosives.
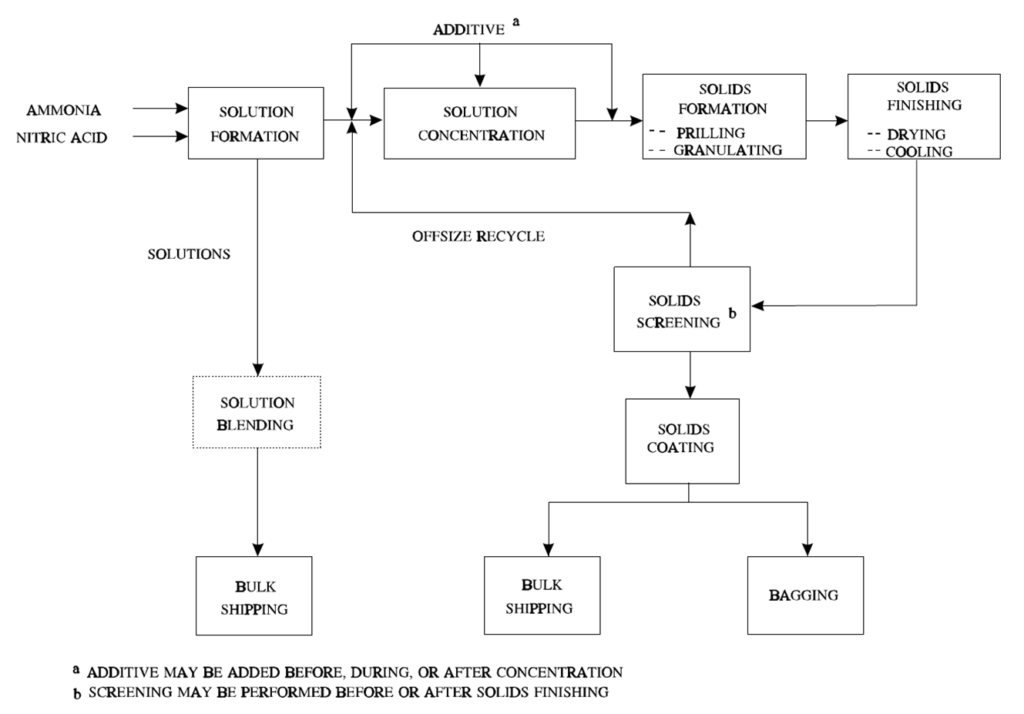
Ammonium nitrate manufacturing operations.
Urea is one of the most widely used nitrogen-based fertilizers due to its high nitrogen content (46% by weight). When applied to soil, urea undergoes chemical transformations to release nitrogen in forms that plants can absorb and use for growth.
How Urea Acts as a Fertilizer
1. Hydrolysis of Urea
Upon application to soil, urea reacts with water in the presence of the enzyme urease, which is naturally present in the soil:
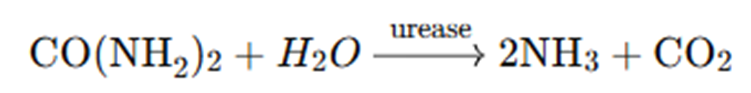
Urea is converted into ammonia and carbon dioxide
Action of urea as fertilizer
2. Ammonia Volatilization
Ammonia gas released during hydrolysis can escape into the atmosphere if not immediately incorporated into the soil.
To minimize losses:
•Urea is incorporated into the soil through tilling
•Urea is applied before rainfall or irrigation.
Note: Urea is highly soluble in water
3. Ammonium Formation
Ammonia reacts with water in the soil to form ammonium ions
NH3 + H2O → NH4+ + OH−
•
Ammonium is positively charged and binds to negatively charged soil particles, making it less prone to leaching.
4. Nitrification
Ammonium is further converted into nitrate through nitrification, a two-step process mediated by soil bacteria:
•
•
•Oxidation of Ammonium to Nitrite:
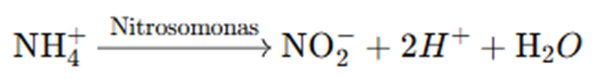
•Oxidation of Nitrite to Nitrate:

Nitrate is the primary form of nitrogen absorbed by plants but is susceptible to leaching if not utilized promptly.
NPK fertilizer
The term “NPK” stands for the three primary nutrients that are vital to plant growth:
N stands for Nitrogen
P stands for Phosphorus
K stands for Potassium
Each of these elements plays a crucial role in plant health:
Understanding NPK Fertilizer Ratios
NPK fertilizers come in different ratios, which specify the percentage of each nutrient present. For example, a fertilizer with an NPK ratio of 10-20-10 contains: 10% nitrogen, 20% phosphorus
and 10% potassium.
•
Different plants and growth stages require different NPK ratios. Here are some examples:
High Nitrogen (30-10-10): Used for leafy vegetables, grasses, or plants requiring a lot of leafy growth.
Balanced Fertilizer (10-10-10): Ideal for general-purpose use across a wide range of plants.
High Phosphorus (10-20-10): Used for promoting root development, flowering, and fruiting, typically used during the planting stage or for flowering plants.
High Potassium (15-15-30): Promotes overall plant health, disease resistance, and strong stems, used for fruiting or flowering plants.
How to Use NPK Fertilizer
The proper application of NPK fertilizers is important to prevent over-fertilization, which can harm plants and the environment. Here’s a quick guide on how to use it:
Soil Testing: Before applying NPK fertilizer, it’s always a good idea to conduct a soil test to understand the existing nutrient levels. This can help avoid nutrient imbalances.
Application Methods: There are several ways to apply NPK fertilizers:
Broadcasting: Spread evenly over the soil surface.
Side-dressing: Applying fertilizer to the soil around the plants.
Foliar Feeding: Spraying a diluted solution on the leaves for quick absorption (typically for micronutrient deficiencies).
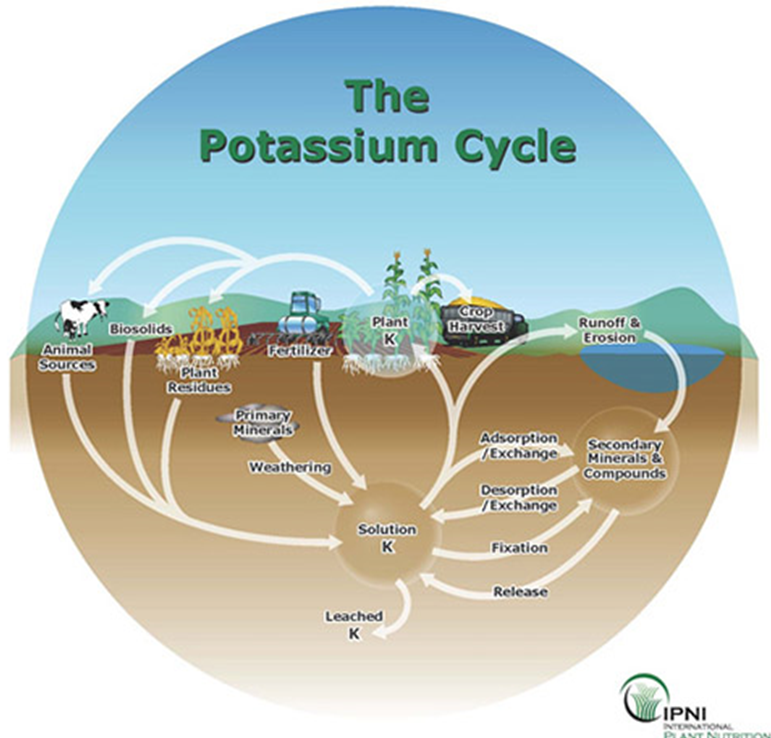
Potassium fertilizer
Sources of potassium
There are a limited number of fertilizer materials that can supply K when needed.
| Material | Chemical formula | Approximate K2O |
| Potassium chloride | KCl | 60 to 62% |
| Potassium sulfate | K2SO4 | 50% |
| Potassium-magnesium sulfate (K-mag or Sul-Po-Mag) | K2SO4·2MgSO4 | 20% |
| Potassium thiosulfate | K2S2O3 | 17% |
| Potassium nitrate | KNO3 | 44% |
1. Single Superphosphate (SSP)
Formula: Ca(H₂PO₄)₂ · H₂O
Content: Contains around 16-22% phosphorus (P₂O₅).
Production: Phosphate rock + sulfuric acid.
2. Triple Superphosphate (TSP)
Formula: Ca(H₂PO₄)₂
Content: Contains around 45-48% phosphorus (P₂O₅).
Production: Phosphate rock + phosphoric acid.
3. Monoammonium Phosphate (MAP)
Formula: NH₄H₂PO₄
Content: Contains 52% phosphorus (P₂O₅) and 11% nitrogen (N).
Production: Phosphoric acid + ammonia.
4. Diammonium Phosphate (DAP)
Formula: (NH₄)₂HPO₄
Content: Contains 46% phosphorus (P₂O₅) and 18% nitrogen (N).
Production: Phosphoric acid + ammonia.
5. Ammonium Polyphosphate (APP)
Formula: (NH₄PO₄)n (where n represents the degree of polymerization)
Content: Varies, generally contains both nitrogen and phosphorus in a water-soluble form.
Production: Phosphoric acid + ammonia under polymerization conditions.
Phosphorus Availability and Soil Chemistry
•Soil-Phosphorus Interactions
•Phosphorus Fixation: Binding with calcium, iron, and aluminum in different soil types.
•Phosphorus in Alkaline Soils: Precipitation with calcium.
•Phosphorus in Acidic Soils: Precipitation with iron and aluminum.
•Soil pH and Fertilizer Efficiency: Optimal pH ranges for phosphorus availability.
•Phosphorus Sorption and Desorption
•Impact of soil texture, organic matter, and microbial activity.
•Influence of soil amendments (lime, organic matter, etc.) on phosphorus availability.
pH 6.5–7.5: Best for phosphorus availability.
Below pH 6: Phosphorus may become fixed with iron and aluminum, reducing availability.
Above pH 7.5: Phosphorus may precipitate with calcium, reducing availability
